Analysis of differentially expressed genes and drug targets based on preoperative and postoperative muscle tissue of obese patients with Roux-en-Y gastric bypass
-
摘要:目的
分析Roux-en-Y胃旁路术(RYGB)对肥胖症患者肌肉组织基因表达的影响, 探讨肥胖症的潜在治疗靶点。
方法筛选GEO数据库,下载GSE161643数据集,获得RYGB术后肥胖症患者肌肉组织的差异表达基因,进行富集分析和免疫细胞浸润分析,构建靶点-化合物相互作用网络,筛选出关键靶点,并利用CMap数据库寻找可治疗肥胖症的潜在候选药物。
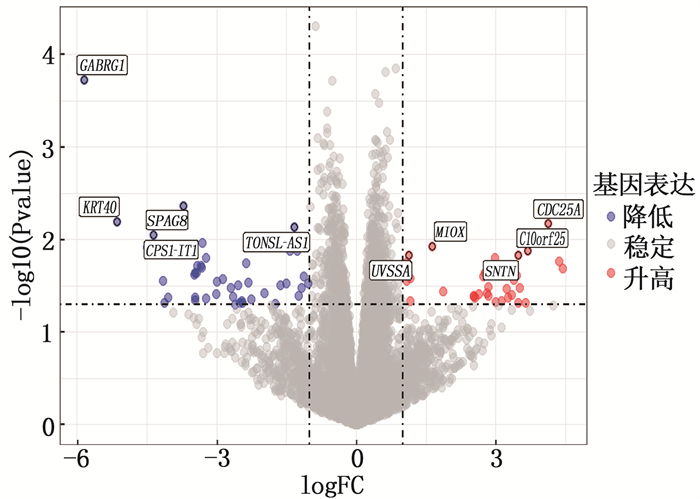
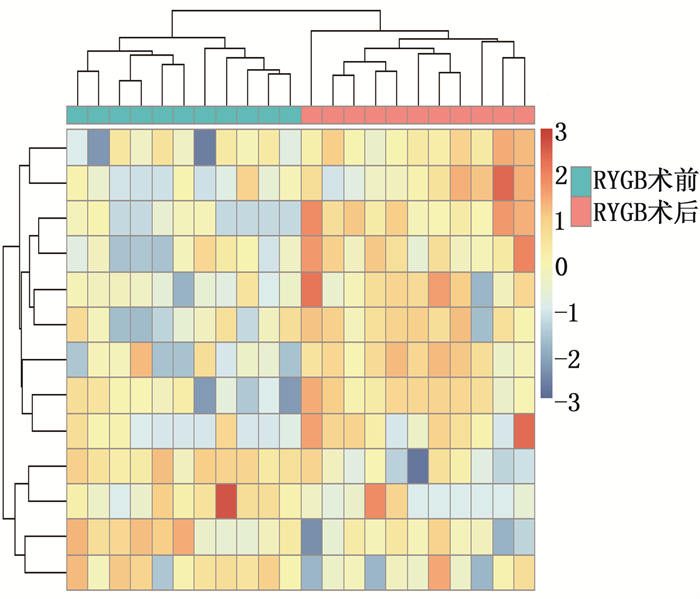
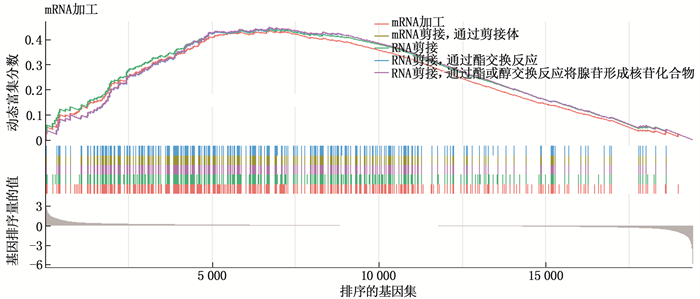
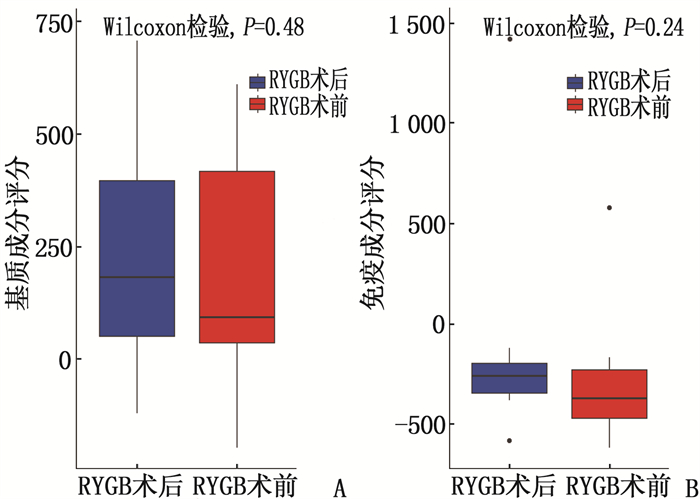
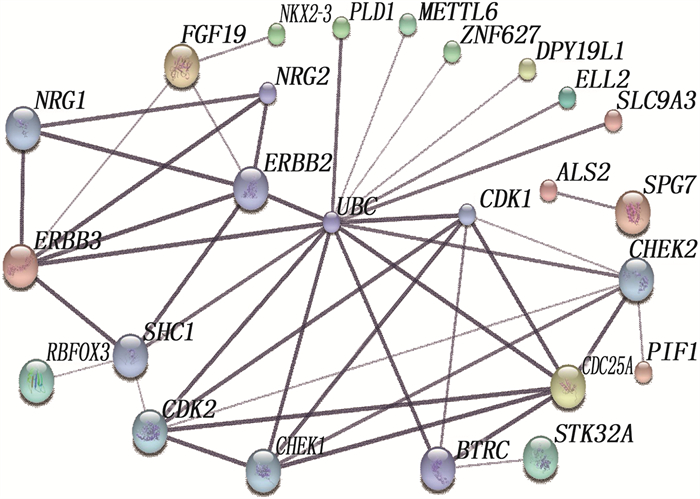
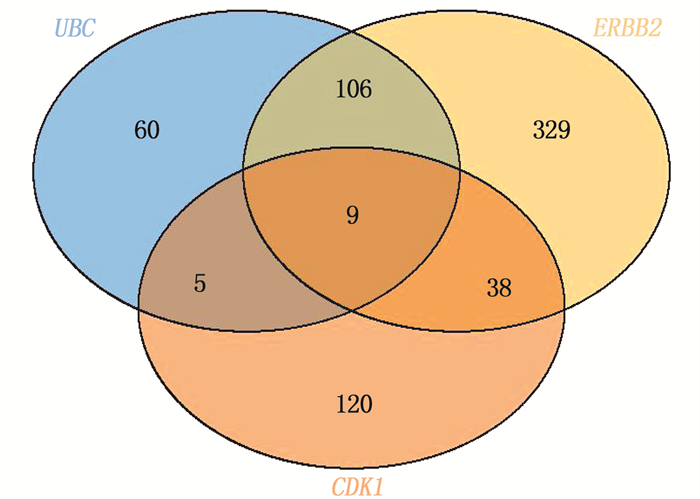
结果本研究共筛选出RYGB术后肌肉组织差异表达基因74个,其中上调基因30个、下调基因44个。基因集富集分析结果提示,GSE161643数据集富集于mRNA加工、RNA剪接、mRNA加工的调控、RNA剪接的调控等8个GO功能。京都基因与基因组百科全书(KEGG)富集分析显示,GSE161643数据集富集于单纯疱疹病毒1型感染通路。RYGB术后,肌肉组织样本的基质成分评分、免疫成分评分与RYGB术前比较,差异均无统计学意义(P>0.05)。靶点-化合物相互作用网络共筛选出10个关键靶点,分别为UBC、CDK1、ERBB2、CDK2、CHEK2、CDC25A、ERBB3、SHC1、CHEK1和BTRC。CMap数据库共筛选出共同作用于UBC、CDK1、ERBB2的小分子化合物9个,分别为氯吡格雷、BNTX、香豆素、左氧氟沙星、SID-26681509、依折麦布、异丁香酚、渥曼青霉素和PSB-06126。
结论与RYGB术前相比,肥胖症患者RYGB术后的肌肉组织基因表达存在显著差异,这些差异可能成为治疗肥胖症的潜在药物靶点。
Abstract:ObjectiveTo analyze effect of expression of muscle tissue of obese patients with Roux-en-Y gastric bypass(RYGB), and to explore potential therapeutic targets for obesity.
MethodsThe dataset GSE16164 was downloaded from the Gene Expression Omnibus (GEO) database, thereby obtaining differentially expressed genes in muscle tissue of obese patients after RYGB. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) were performed to analyze biological functions and pathways. Enrichment analysis and immune cell infiltration analysis were performed to construct target-compound interaction network, key targets were screened out, and potential drug candidates for obesity treatment were found out by using CMap database.
ResultsThere were 74 differentially expressed genes screened in the dataset GSE16164, including 30 up-regulated genes and 44 down-regulated genes. The gene enrichment analysis showed that dataset GSE16164 was mainly involved in 8 gene ontology functions, such as mRNA processing, RNA splicing, regulation of mRNA processing, regulation of RNA splicing, and so forth. KEGG pathway enrichment suggestedthat the dataset GSE161643 was enriched in pathway of herpes simplex virus 1 infection. There were no significant differences in the matrix component score and immune component score of muscle tissue samples after RYGB operation compared with that before RYGB (P>0.05). A total of 10 key targets were screened by target-compound interaction network, including UBC, CDK1, ERBB2, CDK2, CHEK2, CDC25A, ERBB3, SHC1, CHEK1 and BTRC. A total of 9 small molecular compounds targeted on UBC, CDK1, and ERBB2 were screened out in CMap database, which were clopidogrel, BNTX, cymarin, levofloxacin, SID-26681509, ezetimibe, isoeugenol, wortmannin, and PSB-06126.
ConclusionThere are significant differences in gene expressions of skeletal muscle tissue of obese patients with RYGB postoperatively compared with before RYGB operation. These differences maybe serve as potential pharmacological targets for the therapeutic agents of obesity.
-
Keywords:
- obesity /
- Roux-en-Y gastric bypass /
- muscle tissue /
- differentially expressed genes /
- bioinformatics /
- drug targets
-
绒毛膜癌(CC)简称绒癌,属于高度恶性滋养细胞肿瘤,有50%的妊娠CC发生在葡萄胎之后,其余的发生在自然流产和人工流产、正常足月妊娠或早产以及异位妊娠之后[1-2]。目前常用的化疗药物有甲氨蝶呤、环磷酞胺等,但这些化疗药物毒副作用大。多西他赛与紫杉醇都属于紫杉醇类药物,但多西他赛抗肿瘤活性更高,抗癌谱更广,其机制是加强微管蛋白聚合作用和抑制微管解聚作用,导致形成稳定的非功能性微管束,因而破坏肿瘤细胞的有丝分裂[3], 在临床中被广泛应用于治疗难治性卵巢癌等[4-7]。作者的前期体外实验[8]证实多西他赛能抑制CC细胞的增殖,诱导细胞凋亡并明显抑制其侵袭能力。研究[9]报道多西他赛能降低对常规化疗药物不敏感的CC患者血清人绒毛膜促性腺激素(HCG)水平,但多西他赛治疗CC的作用机制还缺乏深入研究。网络药理学融合了系统生物学、多向药理学和计算机信息学等多门学科,建立“药物-成分-基因-靶点-通路-疾病”的多层次网络关系,探索天然药物与复杂疾病的相互关系[10-11]。本研究基于前期实验研究和临床文献结果,采用网络药理学手段探索多西他赛治疗CC的潜在作用机制,现将结果报告如下。
1. 材料与方法
1.1 多西他赛、CC的靶点收集
在Genecards数据库(https://www.genecards.org/)中分别以“Docetaxol” “choriocarcinoma”为关键词检索靶点,并将其导入Excel表格中进行标准化处理,进行统计分析。
1.2 关键靶点筛选
将多西他赛所对应的靶点基因和CC相关的靶点基因进行映射,获得两者的共同基因,即多西他赛治疗CC的作用靶点。
1.3 蛋白质互作(PPI)网络构建
将筛选出的基因输入String 11.0数据库,将蛋白种类设置为人类(homo sapiens), “minimum required interaction score”参数设置为“highest confidence(0.900)”, 其余参数保持初始默认设置,导出PPI数据文件,建立蛋白与蛋白之间的网络。将结果导入Cytoscape软件,对结果进行可视化。
1.4 Hub基因筛选
通过Cytoscape软件的Cytohubba插件中的MCC算法,计算并筛选排名前10位的多西他赛治疗CC的Hub基因。
1.5 基因本体论(GO)富集和京都基因和基因组百科全书(KEGG)通路富集
将共同基因输入DAVID 6.7数据库(http://david.ncifcrf.gov/)进行GO富集分析和KEGG通路分析,研究多西他赛治疗CC的关键靶点的生物功能以及参与的信号通路,以P < 0.05和错误发现率(FDR) < 0.05作为筛选条件,筛选具有显著差异的生物过程和靶点通路。
2. 结果
2.1 靶点蛋白PPI网络的构建
在Genecards数据库分别以“Docetaxol”和“choriocarcinoma”为关键词检索靶点,分别获得953和1 137个靶点,将1 137个疾病靶点与953个药物靶点输入Venny2.1软件绘制韦恩图,筛选出294个共同靶点,见图 1。将共同靶点在String 11.0平台分析的数据导入Cytoscape3.6.1软件,构建PPI网络模型。以Combined_score>0.9为筛选条件绘制PPI网络图,其中节点为靶点蛋白,边为各蛋白间互作关系,节点连接边数越多代表其在网络中作用越重要,见图 2。通过Cytoscape软件插件Cytohubba计算排名前10位的Hub基因,其中TP53、AKT1、CASP8、STAT3等是多西他赛治疗CC的关键基因。见图 3。
2.2 GO功能和KEGG富集分析
将294个预测靶点导入DAVID数据库中进行GO富集分析和KEGG通路分析。GO富集分析中,参与的生物过程有409个,主要包括调控细胞增殖(细胞增殖调节)、细胞凋亡(细胞凋亡负调控、细胞死亡的调控、程序性细胞死亡的调控)、信号传导(信号传导的正调控)、调节分子代谢(大分子代谢及合成过程的正调控、有机物质的反应等)。KEGG通路富集分析确定了28条相关信号通路,主要涉及癌症通路、细胞凋亡通路、Focal Adhesion通路和p53、ErbB、MAPK等信号通路。见图 4、图 5。
3. 讨论
CC属于恶性滋养细胞癌,是由滋养层滋养细胞增殖引起的恶性转化,导致这种转化的分子机制尚未确定。CC可继发于葡萄胎妊娠,也可继发于非葡萄胎妊娠,病情发展迅速,病死率高,主要通过血行播散,转移早且易扩散,肺是CC最易转移的部位,其次为阴道、盆腔等,转移部位皆有局部出血灶[1, 12]。
多西他赛也称多西紫杉醇,是紫杉醇类化学药物的衍生物,临床应用广泛,常被用于治疗难治性卵巢癌等,但其应用于CC方面的研究较少[4-6]。作者前期实验研究发现,多西他赛可以抑制CC细胞增殖的能力,并能诱导CC细胞凋亡,其作用浓度与时间呈正相关,并能明显抑制CC细胞体外侵袭的能力[8]。对高度化疗耐药的CC细胞,每周使用单剂多西他赛可快速降低HCG水平(超过95%), 且对人体毒性最小,但其作用机制有待进一步深入研究。
本研究结果发现,多西他赛可靶向P53、AKT1、STAT3、VEGFA等294个基因,参与细胞凋亡与增殖的调控、分子合成与代谢的调控等主要生物过程。抑癌基因TP53编码p53肿瘤蛋白,其能调节细胞周期和避免细胞癌变发生。任何导致TP53失活的突变都会引起其抑癌功能的丧失而导致癌症发生[13]。研究[14]发现女性TP53突变携带者罹患CC的风险更高,且易产生化疗耐药,因此靶向调控p53对于多西他赛治疗CC具有重要的临床价值。蛋白激酶B1(AKT1)是AKT的一个亚型,是生存信号通路PI3K/AKT的关键分子,与肿瘤细胞的生长、增殖、促进细胞侵袭和转移以及促进血管形成密切相关。研究[15]发现,在CC细胞系中,逆转AKT1表达可能通过抑制PI3K/AKT信号通路及靶向负调控VEGFA表达,最终抑制CC细胞增殖、迁移和侵袭。信号转导与转录激活因子3(STAT3)是一类具有信号转导及转录调控双重功能的蛋白质家族,激活后与DNA分子结合发挥作用[16]。多项研究[17-18]证实STAT3是参与恶性细胞转化的信号转导介质, IL-6/STAT3通路与CC细胞增殖密切相关,阻断STAT3信号通路能够抑制CC细胞的增殖,并诱导CC细胞凋亡。
研究[19-20]表明, p53信号通路与肿瘤发展及预后密切相关,其可以激活多条信号通路,阻碍细胞周期,诱导细胞凋亡,促进细胞衰老以及DNA修复过程,最终抑制肿瘤生长。研究[21]发现p53和p21CIP1是p53信号通路中重要的靶基因,在CC JEG-3细胞中,细胞周期蛋白依懒性激酶抑制剂p21CIPI表达被激活后,使G2细胞周期蛋白依赖性激酶CDK1表达显著降低,同时p53丝氨酸磷酸化水平增加, p53信号通路激活后使细胞周期阻滞在G2/M期,进而激活DNA损伤反应通路,抑制细胞增殖。
本研究借助网络药理学方法对多西他赛和CC进行靶标挖掘和通路分析,得到294个共同靶向基因和CC多条信号通路,包括TP53、AKT1、STAT3、VEGAF等基因以及细胞凋亡通路、p53通路等信号通路,证明了多西他赛可能通过调节多个靶基因、调控蛋白表达和信号传导通路而发挥对CC的治疗作用,初步揭示了多西他赛治疗CC的作用机制,为后续临床研究提供了新思路。
-
表 1 共同作用于UBC、CDK1、ERBB2的小分子化合物
序号 预测分数 名称 类型 靶点 1 98.33 氯吡格雷 嘌呤受体拮抗剂 P2RY12, CYP2B6, CYP2C19, CYP3A5 2 96.30 BNTX 阿片类受体拮抗剂 OPRD1, OPRK1, OPRM1 3 94.36 香豆素 ATP酶抑制剂 ATP1A1 4 89.09 左氧氟沙星 细菌DNA回旋酶抑制剂 TOP2A 5 89.05 SID-26681509 胰蛋白酶抑制剂 CTSL 6 81.01 依泽米贝 NPC1L1蛋白拮抗剂 NPC1L1, ANPEP, SOAT1 7 81.00 异丁香酚 一氧化氮生成抑制剂 — 8 80.99 渥曼青霉素 PI3K抑制剂 PIK3CA, PIK3CG, PLK1, ATM, ATR, MTOR, PI4KA, PI4KB, PIK3CD, PIK3R1, PLK3, PRKDC 9 80.82 PSB-06126 NTPD酶抑制剂 ENTPD3 -
[1] PUHL R, SUH Y. Health consequences of weight Stigma: implications for obesity prevention and treatment[J]. Curr Obes Rep, 2015, 4(2): 182-190. doi: 10.1007/s13679-015-0153-z
[2] 陈玉, 周铃, 徐耀初, 等. 肥胖与糖尿病患病关系的调查研究[J]. 江苏临床医学杂志, 2000, 4(2): 110-112. https://www.cnki.com.cn/Article/CJFDTOTAL-XYZL200002012.htm [3] 李梦伊, 刘洋, 赵象文, 等. 大华北减重与代谢手术临床资料数据库2019年度报告[J]. 中国实用外科杂志, 2020, 40(4): 418-425. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202004018.htm [4] 杨景哥, 王存川, 胡友主, 等. 腹腔镜Roux-en-Y胃旁路手术治疗肥胖症和2型糖尿病[J]. 中华胃肠外科杂志, 2010, 13(8): 594-597. doi: 10.3760/cma.j.issn.1671-0274.2010.08.014 [5] 焦宇文, 汤黎明, 钱峻, 等. 腹腔镜袖状胃切除对肥胖患者炎症因子水平的影响[J]. 实用临床医药杂志, 2018, 22(22): 103-104, 107. doi: 10.7619/jcmp.201822034 [6] MADSEN L R, BAGGESEN L M, RICHELSEN B, et al. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: a Danish population-based matched cohort study[J]. Diabetologia, 2019, 62(4): 611-620. doi: 10.1007/s00125-019-4816-2
[7] 闫文貌, 靖长友, 李有国, 等. 胃旁路术对2型糖尿病患者的三年疗效分析[J]. 中华腔镜外科杂志: 电子版, 2018, 11(5): 268-273. doi: 10.3877/cma.j.issn.1674-6899.2018.05.003 [8] COLETTA D K, MANDARINO L J. Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria[J]. Am J Physiol Endocrinol Metab, 2011, 301(5): E749-E755. doi: 10.1152/ajpendo.00363.2011
[9] NG M, FLEMING T, ROBINSON M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013[J]. Lancet, 2014, 384(9945): 766-781. doi: 10.1016/S0140-6736(14)60460-8
[10] NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults[J]. Lancet, 2017, 390(10113): 2627-2642. doi: 10.1016/S0140-6736(17)32129-3
[11] CHUNG E, OFFEI S D, JIA U T A, et al. A synthesis of a rationally designed inhibitor of cytochrome P450 8B1, a therapeutic target to treat obesity[J]. Steroids, 2022, 178: 108952. doi: 10.1016/j.steroids.2021.108952
[12] WITTGROVE A C, CLARK G W, TREMBLAY L J. Laparoscopic gastric bypass, roux-en-Y: preliminary report of five cases[J]. Obes Surg, 1994, 4(4): 353-357. doi: 10.1381/096089294765558331
[13] GASTALDI G, RUSSELL A, GOLAY A, et al. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure[J]. Diabetologia, 2007, 50(11): 2348-2355. doi: 10.1007/s00125-007-0782-1
[14] LEICHMAN J G, WILSON E B, SCARBOROUGH T, et al. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery[J]. Am J Med, 2008, 121(11): 966-973. doi: 10.1016/j.amjmed.2008.06.033
[15] ALBERS P H, BOJSEN-MØLLER K N, DIRKSEN C, et al. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery[J]. Am J Physiol Regul Integr Comp Physiol, 2015, 309(5): R510-R524. doi: 10.1152/ajpregu.00228.2014
[16] TAMBOLI R A, HAJRI T, JIANG A X, et al. Reduction in inflammatory gene expression in skeletal muscle from Roux-en-Y gastric bypass patients randomized to omentectomy[J]. PLoS One, 2011, 6(12): e28577. doi: 10.1371/journal.pone.0028577
[17] CAMPBELL L E, LANGLAIS P R, DAY S E, et al. Identification of novel changes in human skeletal muscle proteome after roux-en-Y gastric bypass surgery[J]. Diabetes, 2016, 65(9): 2724-2731. doi: 10.2337/db16-0004
[18] LIU L, XIE B W, FAN M, et al. Low-level saturated fatty acid palmitate benefits liver cells by boosting mitochondrial metabolism via CDK1-SIRT3-CPT2 cascade[J]. Dev Cell, 2020, 52(2): 196-209, e9. doi: 10.1016/j.devcel.2019.11.012
[19] SHANG W T, SI X, ZHOU Z K, et al. Wheat bran with enriched gamma-aminobutyric acid attenuates glucose intolerance and hyperinsulinemia induced by a high-fat diet[J]. Food Funct, 2018, 9(5): 2820-2828. doi: 10.1039/C8FO00331A
[20] FULLER S J, SIVARAJAH K, SUGDEN P H. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium[J]. J Mol Cell Cardiol, 2008, 44(5): 831-854. doi: 10.1016/j.yjmcc.2008.02.278
[21] MO J L, CHEN Z M, XU J, et al. Efficacy of clopidogrel-aspirin therapy for stroke does not exist in C YP2C19 loss-of-function allele noncarriers with overweight/obesity[J]. Stroke, 2020, 51(1): 224-231. doi: 10.1161/STROKEAHA.119.026845
[22] SUN Y F, VENUGOPAL J, GUO C A, et al. Clopidogrel resistance in a murine model of diet-induced obesity is mediated by the interleukin-1 receptor and overcome with DT-678[J]. Arterioscler Thromb Vasc Biol, 2020, 40(6): 1533-1542. doi: 10.1161/ATVBAHA.120.314146
[23] YAO H W, GU J K, SHAN Y Q, et al. Type 2 diabetes mellitus decreases systemic exposure of clopidogrel active metabolite through upregulation of P-glycoprotein in rats[J]. Biochem Pharmacol, 2020, 180: 114142. doi: 10.1016/j.bcp.2020.114142
[24] LEE Y S, PARK J S, LEE D H, et al. Ezetimibe ameliorates lipid accumulation during adipogenesis by regulating the AMPK-mTORC1 pathway[J]. FASEB J, 2020, 34(1): 898-911. doi: 10.1096/fj.201901569R
[25] CHO Y, KIM R H, PARK H, et al. Effect of ezetimibe on glucose metabolism and inflammatory markers in adipose tissue[J]. Biomedicines, 2020, 8(11): 512. doi: 10.3390/biomedicines8110512
[26] SWIDERSKA E, STRYCHARZ J, WRÓBLEWSKI A, et al. Chronic and intermittent hyperglycemia modulates expression of key molecules of PI3K/AKT pathway in differentiating human visceral adipocytes[J]. Int J Mol Sci, 2021, 22(14): 7712. doi: 10.3390/ijms22147712
[27] JIN J, CHENG R Y, REN Y, et al. Distinctive gut microbiota in patients with overweight and obesity with dyslipidemia and its responses to long-term orlistat and ezetimibe intervention: a randomized controlled open-label trial[J]. Front Pharmacol, 2021, 12: 732541. doi: 10.3389/fphar.2021.732541
[28] DE BRITO B B, DA SILVA F A F, SOARES A S, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection[J]. World J Gastroenterol, 2019, 25(37): 5578-5589. doi: 10.3748/wjg.v25.i37.5578
[29] MARTÍN-NÙÑEZ G M, CORNEJO-PAREJA I, ROCA-RODRÍGUEZ M, et al. H. pylori eradication treatment causes alterations in the gut microbiota and blood lipid levels[J]. Front Med (Lausanne), 2020, 7: 417.
[30] GUTIÉRREZ-REPISO C, MORENO-INDIAS I, MARTÍN-NÙÑEZ G M, et al. Influence of factors altering gastric microbiota on bariatric surgery metabolic outcomes[J]. Microbiol Spectr, 2021, 9(3): e0053521. doi: 10.1128/Spectrum.00535-21
[31] ANG T L, LIM K W, ANG D, et al. Clinical audit of current Helicobacter pylori treatment outcomes in Singapore[J]. Singapore Med J, 2021: 2021Sep21.
-
期刊类型引用(1)
1. 卢俊伟,祝璟哲,陈鸿儒,解举民. 基于网络药理学与分子对接技术探究木犀草素治疗宫颈癌的分子机制. 实用临床医药杂志. 2024(16): 26-33 .  本站查看
本站查看
其他类型引用(1)





 下载:
下载:




 苏公网安备 32100302010246号
苏公网安备 32100302010246号
